CLINICAL TRIALS
JOINT HEALTH
1. BioCell Collagen on subjective pain caused by various joint discomforts
- This trial enrolled 89 subjects who were experiencing pain caused by joint discomfort.
- The subjects ingested 2 grams daily of BioCell Collagen for 45 days.
- Out of 89, 80 subjects (89%) taking BioCell Collagen experienced a degree of improved joint comfort.
- In contrast, only one subject who took placebo had improved joint comfort.
- No adverse events associated with BioCell Collagen were reported.
Results of study – Effective in 89% of subjects
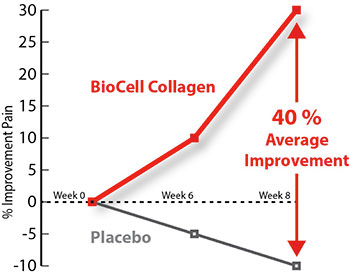
2. BioCell Collagen on joint discomfort
-
This randomized, double-blind, and placebo-controlled trial enrolled 16 subjects who had joint discomfort.
- The safety and efficacy of BioCell Collagen in managing joint discomfort was investigated by using the WOMAC index.
- The subjects ingested 2 grams daily of BioCell Collagen for 8 weeks.
- As compared to placebo, BioCell Collagen significantly reduced the joint discomfort as much as 40% at the end of the study.
- No adverse events associated with BioCell Collagen were reported.
- The study details were presented at the international conference of Experimental Biology, 2004, Washington, DC.
Results of the study – 40% improvement
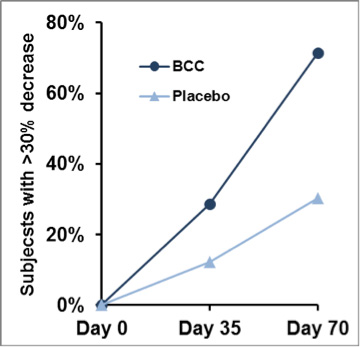
3. BioCell Collagen on joint discomfort – expanded, confirmatory clinical trial
- This randomized, double-blind, and placebo-controlled trial enrolled 80 subjects who had joint discomfort.
- The safety and efficacy of BioCell Collagen in managing joint discomfort was investigated by using the WOMAC index.
- The subjects ingested 2 grams daily of BioCell Collagen for 10 weeks.
- As compared to placebo, BioCell Collagen significantly reduced joint discomfort, confirming the earlier trial discussed above.
- No adverse events associated with BioCell Collagen were reported.
- The study outcome was published in Journal of Agricultural and Food Chemistry (2012).
Results of study – Min. 30% less discomfort in 71% of subjects taking BioCell Collagen
4. BioCell Collagen may improve recovery following weight training exercise
- Blood markers associated with muscular stress, creatine kinase, lactate dehydrogenase, and C-reactive protein, all showed improved levels following exercise challenge(s) between the BioCell Collagen treated and placebo groups
- Post challenge performance decrement was diminished in BioCell Collagen individuals versus the placebo group
The study outcome was published in the Journal of the International Society of Sports Nutrition.
- This study enrolled 26 subjects who were undergoing both natural (chronological) and photo aging process in their face.
- Both qualitative and quantitative tools were used to measure visible parameters such as wrinkles and fine lines and internal parameters such as dermal collagen content, hydration, and blood microcirculation.
- The subjects ingested 1 gram daily of BioCell Collagen for 12 weeks.
- BioCell Collagen demonstrated the following multi-layered mechanisms which counteracted both natural and photoaging signs.
- Significant reduction of facial lines and wrinkles
- Significant reduction of dryness and skin scaling
- Significant increase in collagen content in the skin dermis
- No adverse events associated with BioCell Collagen were reported.
- The study outcome was published in The Clinical Interventions in Aging (2012).
(http://www.ncbi.nlm.nih.gov/pubmed/22956862)
In a hyaluronic acid (HA) bioavailability study, subjects taking Liquid BioCell™ (1500 mg/daily) for 28 days were shown to increase steady-state HA levels in their blood as much as 60-fold (Figure 1).
Figure 1. Increase in steady-state HA levels.

Hyaluronidase is an enzyme that catalyzes the degradation of hyaluronic acid (HA). Liquid BioCell deactivates hyaluronidase, preventing it from destroying HA in the body (Figure 2).
Figure 2. Dose-dependent inhibition of hyaluronidase.

Additional Clinical Studies
1. Sheldon, E “A randomized double blind clinical pilot trial evaluating the safety and efficacy of hydrolyzed collagen type II (BioCell Collagen II®) in adults with osteoarthritis,” Miami Research Associates. April 25, 2003.
Clinical Study Shows Biocell Collagen II® Effective for Osteoarthritis
The findings of Dr. Eric Sheldon, a clinical research investigator at Miami Research Associates, are derived from a placebo-controlled pilot study in sixteen men and women with OA who received the supplement for an eight-week period. The supplement tested is a unique, patented extract of naturally occurring type II Collagen, Chondroitin Sulfate, and Hyaluronic Acid named BioCell Collagen II®.
“This preliminary study suggests that BioCell Collagen II has promise in the management of chronic osteoarthritis symptoms,” said Sheldon, a rheumatologist and voluntary rheumatology instructor at the University of Miami School of Medicine. “We used a symptom assessment tool that is used routinely in OA drug studies and the results are encouraging.” Osteoarthritis, also known as degenerative joint diseases, is the most common form of arthritis.
Sheldon said the data reveal that daily consumption of BioCell Collagen II led to clinically meaningful improvements that were significantly superior to the group receiving placebo supplements. Additionally, the BioCell Collagen II group had no greater incidence of adverse events or side effects.
2. William, Judy, “Clinical study shows hyaluronic acid in BioCell Collagen II® found to have significant absorption and bioavailability,” SIBR. February, 2 2004
Clinical Study published in The FASEB Journal Shows Hyaluronic Acid in Biocell Collagen II® Found To Have Significant Absorption and Bioavailability
Dr. William Judy, senior scientist at SIBR Research, called the double blind clinical study proving that the naturally occurring Hyaluronic Acid (HA) in the BioCell Collagen II product has significant peak absorption and steady state bioavailability in normal volunteer subjects “groundbreaking science” and noted BioCell Technology’s revolutionary form of a reduced molecular weight HA manufactured using patented technology, is absorbed and is therefore readily available for use by the body. This is in contrast to previous published studies which showed that other HA forms were not absorbed and thus unavailable for use by the human body.
In a 36-hour peak absorption study using a single dose, BioCell Collagen II HA significantly increased in the blood in four hours and peaked at a level 7008.62% above control in twelve (12) hours (P=0.05). In the blood, HA was rapidly metabolized to two metabolites 1/600th the size of the ingested HA.
In a 28-day steady state bioavailability study using a constant daily dose, after seven (7) days, BioCell Collagen II HA and its metabolites in the blood became stable, and these metabolites remained significantly increased (p=0.001) throughout the balance of the study (HA at 3542.58% above control and HA metabolite at 11890.15% above control).
By determining the rate and magnitude of HA absorption and its bioavailability, this study using the BioCell Collagen II product clearly demonstrates that this HA form has the physical characteristics necessary to allow it and its metabolites to move rapidly from the blood to the tissues. These findings further support the previous documented efficacy of BioCell Collagen II in the management of joint and skin care. This unprecedented study on hyaluronic acid, a key element in the unique matrix of BioCell Collagen II which consists of Collagen Type II, Chondroitin Sulfate, and Hyaluronic Acid, demonstrates a synergistic effect that allows for the impressive absorption as indicated in these exciting and promising studies.
3. Food Chem Toxicol. 2007 Feb;45(2):315-21. Epub 2006 Aug 30.
Acute and subchronic oral toxicity studies in rats of a hydrolyzed chicken sternal cartilage preparation.
Schauss AG, Merkel DJ, Glaza SM, Sorenson SR.
AIBMR Life Sciences, Inc., Natural and Medical Products Research, 4117 S. Meridian, Puyallup, WA 98373, USA.
Abstract
Two acute and subchronic oral toxicity studies were conducted in rats to evaluate safety of a patented preparation of hydrolyzed chicken sternal cartilage (BioCell Collagen II) containing collagen type II, chondroitin sulfate, and hyaluronic acid. In the acute oral toxicity study, five males and five females of Sprague-Dawley rats were administered a single dose of 5000 mg of the test product per kg body weight and observed for 14 days. All animals survived and exhibited normal body weight gain throughout the study. Macroscopic necropsy examination conducted on day 15 revealed no gross pathological lesions in any of the animals. In the subchronic study, Sprague-Dawley rats (40 males, 40 females) were divided into four same-sex groups (10 animals/group). Animals in each group were administered daily either 0, 30, 300 or 1000 mg of the test product per kg of body weight for over 90 days. All animals survived and showed no significant changes in their body weights and histopathology. Although some differences were observed between the treated and control animals in several parameters, they were generally not dose-related or considered to be of toxicological significance. In conclusion, the results from the two oral toxicity studies with male and female young adult rats indicated that the test preparation from hydrolyzed chicken sternal cartilage collagen (BioCell Collagen II) was well tolerated at all four doses tested.
4. Cellular and Molecular Life Sciences Volume 65, Number 3, 395-413, DOI: 10.1007/s00018-007-7360-z
Hyaluronan synthesis and degradation in cartilage and bone
E. R. Bastow, S. Byers, S. B. Golub, C. E. Clarkin, A. A. Pitsillides and A. J. Fosang
Abstract
Hyaluronan (HA) is a large but simple glycosaminoglycan composed of repeating D- glucuronic acid, beta1-3 linked to N-acetyl-D-glucosamine beta1-4, found in body fluids and tissues, in both intra- and extracellular compartments. Despite its structural simplicity, HA has diverse functions in skeletal biology. In development, HA-rich matrices facilitate migration and condensation of mesenchymal cells, and HA participates in joint cavity formation and longitudinal bone growth. In adult cartilage, HA binding to aggrecan immobilises aggrecan, retaining it at the high concentrations required for compressive resilience. HA also appears to regulate bone remodelling by controlling osteoclast, osteoblast and osteocyte behaviour. The functions of HA depend on its intrinsic properties, which in turn rely on the degree of polymerisation by HA synthases, depolymerisation by hyaluronidases, and interactions with HA- binding proteins. HA synthesis and degradation are closely regulated in skeletal tissues and aberrant synthetic or degradative activity causes disease. The role and regulation of HA synthesis and degradation in cartilage, bone and skeletal development is discussed.
5. IMMUNOMODULATORY AND ANTI-INFLAMMATORY EFFECTS OF CHONDROITIN SULPHATE
Journal of cellular and molecular medicine volume 13, issue 8a, pages 1451–1463, august 2009
Patrick Du Souich1,*, Antonio G. García2, Josep Vergés3, Eulàlia Montell3
Abstract
- Biochemistry of chondrotin sulphate
- Mechanism of action of chondroitin sulphate
- Effect of chondroitin sulphate on the chondrocyte
- Effect of chondroitin sulphate on the synovial membrane
- Effect of chondroitin sulphate on subchondral bone
- Human use of chondroitin sulphate
- Chondrotin sulphate in osteoarthritis
- Chondrotin sulphate in psoriasis
- Chondrotin sulphate in atherosclerosis
- Chondroitin sulphate in IBD
- Chondroitin sulphate in degenerative diseases of the central nervous system (CNS)
- Other autoimmune diseases that may benefit from chondroitin sulphate
Conclusions
Chondroitin sulphate (CS) is a natural glycosaminoglycan present in the extracellular matrix and is formed by the 1–3 linkage of D-glucuronic acid to N- acetylgalactosamine. In chondrocytes, CS diminishes interleukin-1 p (IL-1p)-induced increases in p38 mitogen-activated protein kinase (p38MAPK) and signal-regulated kinase 1/2 (Erk1/2) phosphorylation, and decreases nuclear factor-KB (NF-kB) nuclear translocation and as a consequence, reduces the formation of pro- inflammatory cytokines, IL-1 p and TNF-a, and pro-inflammatory enzymes, such as phospholipase A2 (PLA2), cyclooxygenase 2 (COX-2) and nitric oxide synthase-2 (NOS-2). The mechanism of action of CS explains its beneficial effect on the cartilage, synovial membrane and subchondral bone. On the other hand, in vivo, CS given orally prevents hepatic NF-KB nuclear translocation, suggesting that systemic CS may elicit an anti-inflammatory effect in many tissues besides the articulation. There is preliminary evidence showing that in human beings, CS may be of benefit in other diseases where inflammation is an essential marker, such as psoriasis and atherosclerosis. The review of the literature suggest that CS might also be of interest for the treatment of other diseases with an inflammatory and/or autoimmune character, such as inflammatory bowel disease, degenerative diseases of the central nervous system and stroke, multiple sclerosis and other autoimmune diseases.
6. Curr Med Res Opin. 2006 Nov;22(11):2221-32.
Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: a review of the literature.
Bello AE, Oesser S. University of Illinois College of Medicine at Chicago, Chicago, IL 60612
ABSTRACT
BACKGROUND: There is a need for an effective treatment for the millions of people in the United States with osteoarthritis (OA), a degenerative joint disease. The demand for treatments, both traditional and non-traditional, will continue to grow as the population ages.
SCOPE: This article reviews the medical literature on the preclinical and clinical research on a unique compound, collagen hydrolysate. Articles were obtained through searches of the PubMed database (www.pubmed.gov) through May 2006 using several pairs of key words (collagen hydrolysate and osteoarthritis; collagen hydrolysate and cartilage; collagen hydrolysate and chondrocytes; collagen hydrolysate and clinical trial) without date limits. In addition, other sources of information, such as abstracts presented at scientific congresses and articles in the German medical literature not available on PubMed, were reviewed and included based on the authors’ judgment of their relevance to the topic of the review.
FINDINGS: According to published research, orally administered collagen hydrolysate has been shown to be absorbed intestinally and to accumulate in cartilage. Collagen hydrolysate ingestion stimulates a statistically significant increase in synthesis of extracellular matrix macromolecules by chondrocytes (p < 0.05 compared with untreated controls). These findings suggest mechanisms that might help patients affected by joint disorders such as OA. Four open-label and three double-blind studies were identified and reviewed; although many of these studies did not provide key information--such as the statistical significance of the findings--they showed collagen hydrolysate to be safe and to provide improvement in some measures of pain and function in some men and women with OA or other arthritic conditions.
CONCLUSION: A growing body of evidence provides a rationale for the use of collagen hydrolysate for patients with OA. It is hoped that ongoing and future research will clarify how collagen hydrolysate provides its clinical effects and determine which populations are most appropriate for treatment with this supplement.
7. Semin Arthritis Rheum. 2000 Oct;30(2):87-99. Role of collagen hydrolysate in bone and joint disease.
Moskowitz RW. Case Western Reserve University, Division of Rheumatic Diseases, University Hospitals of Cleveland, OH, USA.
ABSTRACT
OBJECTIVES: To review the current status of collagen hydrolysate in the treatment of osteoarthritis and osteoporosis.
METHODS: Review of past and current literature relative to collagen hydrolysate metabolism, and assessment of clinical investigations of therapeutic trials in osteoarthritis and osteoporosis.
RESULTS: Hydrolyzed gelatin products have long been used in pharmaceuticals and foods; these products are generally recognized as safe food products by regulatory agencies. Pharmaceutical-grade collagen hydrolysate (PCH) is obtained by hydrolysis of pharmaceutical gelatin. Clinical studies suggest that the ingestion of 10 g PCH daily reduces pain in patients with osteoarthritis of the knee or hip; blood concentration of hydroxyproline is increased. Clinical use is associated with minimal adverse effects, mainly gastrointestinal, characterized by fullness or unpleasant taste. In a multicenter, randomized, doubleblind, placebo-controlled trial performed in clinics in the United States, United Kingdom, and Germany, results showed no statistically significant differences for the total study group (all sites) for differences of mean pain score for pain. There was, however, a significant treatment advantage of PCH over placebo in German sites. In addition, increased efficacy for PCH as compared to placebo was observed in the overall study population amongst patients with more severe symptomatology at study onset. Preferential accumulation of 14C- labeled gelatin hydrolysate in cartilage as compared with administration of 14C- labeled proline has been reported. This preferential uptake by cartilage suggests that PCH may have a salutary effect on cartilage metabolism. Given the important role for collagen in bone structure, the effect of PCH on bone metabolism in osteoporotic persons has been evaluated. Studies of the effects of calcitonin with and without a collagen hydrolysate-rich diet suggested that calcitonin plus PCH had a greater effect in inhibiting bone collagen breakdown than calcitonin alone, as characterized by a fall in levels of urinary pyridinoline cross-links. PCH appeared to have an additive effect relative to use of calcitonin alone.
CONCLUSIONS: Collagen hydrolysate is of interest as a therapeutic agent of potential utility in the treatment of osteoarthritis and osteoporosis. Its high level of safety makes it attractive as an agent for long-term use in these chronic disorders.
8. Osteoarthritis Cartilage. 1998 May;6 Suppl A:14-21.
ANTI-INFLAMMATORY ACTIVITY OF CHONDROITIN SULFATE.
Ronca F, Palmieri L, Panicucci P, Ronca G. -Department of Human and Environmental Sciences, University of Pisa, Italy.
ABSTRACT
The pharmacokinetics of chondroitin sulfate (CS, Condrosulf, IBSA, Lugano, Switzerland) were investigated in rats and in healthy volunteers using CS tritiated at the reducing end and CS labeled with 131I or 99mTc respectively. A rapid absorption of orally administered CS is observed in rats and in humans when the drug is dissolved in water. Lower and delayed absorption is observed when CS is administered in gastroresistant capsules. The absolute bio-availability is 15 and 12% for rats and humans respectively. The CS shows a tropism for cartilagineous tissues in rats and for knee tissues in humans as demonstrated by scintigraphic analysis with 99mTc-CS. Monomers, oligo and polysaccharides produced by enzymatic hydrolysis of CS appear in the blood and tissues together with native CS. The effects of partially depolymerized (m.m. 3 to 15 kD) and desulfated fractions on human leukocytes were investigated. CS and its fractions inhibit the directional chemotaxis induced by zymosan-activated serum, are able to decrease the phagocytosis and the release of lysozyme induced by zymosan and to protect the plasma membrane from oxygen reactive species. In rats the oral administration of CS significantly decreases granuloma formation due to sponge implants and cell migration and lysosomal enzyme release in carrageenan pleurisy. Compared with nonsteroidal anti- inflammatory drugs (indomethacin, ibuprofen), CS appears to be more effective on cellular events of inflammation than on edema formation. It is noteworthy that CS is devoid of dangerous effects on the stomach, platelets and kidneys. In synovial fluid of patients requiring joint aspiration, treated orally for 10 days with CS (800 mg/day) the hyaluronate concentration and the intrinsic viscosity significantly increased, while collagenolytic activity, phospholipase A2 and N-acetylglucosaminidase (NAG) decreased. These results give an insight into the mechanism of the anti-inflammatory and chondroprotective actions demonstrated by this drug in a number of clinical trials in patients with osteoarthritis.
9. Osteoarthritis Cartilage. 2008;16 Suppl 3:S19-21. Epub 2008 Jul 31. Clinical review of chondroitin sulfate in osteoarthritis.
Uebelhart D.–Department of Rheumatology and Institute of Physical Medicine, University Hospital Zurich, Switzerland. daniel.uebelhart@usz.ch
Abstract
Symptomatic slow-acting drugs for the treatment of osteoarthritis (SYSADOA; OA) are compounds which are prescribed as drugs in European countries since many years, whereas they are sold as nutraceuticals in USA. In Europe, the publication of the EULAR Recommendations for the Treatment of Knee OA in 2003 has listed oral chondroitin sulfate (CS) as evidence 1A and strength of recommendation A which represents the highest level for a therapeutic strategy. Symptomatic slow-acting drugs are intended to be used as ground therapy for OA; these compounds are not rapidly acting agents such as Non Steroidal Anti-Inflammatory Drugs (NSAIDs), and their clinical efficacy on algo-functional symptoms can only be demonstrated after a couple of weeks of regular intake. Interestingly, once the administration is stopped, they do show carry-over effects of various durations, from about 3 months with the oral formulations to 6-9 months with intra-articular formulations. The main rationale behind the use of the SYSADOA therapeutic class is the reduction of NSAIDs in the overall drug management of OA disease and therefore consequently to limit the very significant risks of upper Gastro-intestinal (GI) tract erosions, ulcers with bleeding and/or deleterious renal effects in elderly patients. The evidence for clinical efficacy of oral CS as a drug able to significantly improve the algo-functional symptoms of OA disease does come from a set of randomized clinical studies published a couple of years ago. Indeed, it was demonstrated that the drug was effective in knee and finger OA, whereas previous data suggested that hip OA patients could also benefit from it. In addition, oral CS supported the comparison with NSAIDs such as diclofenac sodium in a medium/long-term clinical study in patients with knee OA. A dose- finding study in patients with knee OA did provide strong data supporting the administration of 800 mg of CS orally which had nearly the same effects as 1200 mg/day, whereas the use of a sequential 3 months administration mode, twice a year was also shown to provide the same results as a continuous treatment. The good tolerability and safety aspects of oral CS were largely documented in these CTs. Taking these important points into account, we definitively have enough clinical data available supporting the view that oral CS is a valuable and safe symptomatic treatment for OA disease. More recent data based on a couple of previous trials and two pivotal studies do provide further evidence that oral CS does also have structure- modifying effects in knee OA patients. A couple of other compounds such as hyaluronan, diacerein, avocado and soya unsaponifiables, doxycycline have also been tested with respect to their potential disease-modifying effects. Additional compounds including receptor activator of NF-kappaB (RANK) ligand inhibitors, cathepsin K inhibitors, bisphosphonates are further assessed regarding their potential structure- modifying effect.
10. Am J Pathol. 2009 Jan;174(1):101-14. Epub 2008 Dec 30.
Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin.
Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ. –Department of Dermatology, Medical School, University of Michigan, Ann Arbor, Michigan 48109-5609, USA.
Abstract
Aged human skin is fragile because of fragmentation and loss of type I collagen fibrils, which confer strength and resiliency. We report here that dermal fibroblasts express increased levels of collagen-degrading matrix metalloproteinases-1 (MMP-1) in aged (>80 years old) compared with young (21 to 30 years old) human skin in vivo. Transcription factor AP-1 and alpha2beta1 integrin, which are key regulators of MMP-1 expression, are also elevated in fibroblasts in aged human skin in vivo. MMP- 1 treatment of young skin in organ culture causes fragmentation of collagen fibrils and reduces fibroblast stretch, consistent with reduced mechanical tension, as observed in aged human skin. Limited fragmentation of three-dimensional collagen lattices with exogenous MMP-1 also reduces fibroblast stretch and mechanical tension. Furthermore, fibroblasts cultured in fragmented collagen lattices express elevated levels of MMP-1, AP-1, and alpha2beta1 integrin. Importantly, culture in fragmented collagen raises intracellular oxidant levels and treatment with antioxidant MitoQ(10) significantly reduces MMP-1 expression. These data identify positive feedback regulation that couples age-dependent MMP-1-catalyzed collagen fragmentation and oxidative stress. We propose that this self perpetuating cycle promotes human skin aging. These data extend the current understanding of the oxidative theory of aging beyond a cellular-centric view to include extracellular matrix and the critical role that connective tissue microenvironment plays in the biology of aging.
11. Am J Pathol. 2007 Nov;171(5):1451-61.
Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases.
Dai G, Freudenberger T, Zipper P, Melchior A, Grether-Beck S, Rabausch B, de Groot J, Twarock S, Hanenberg H, Homey B, Krutmann J, Reifenberger J, Fischer JW., Molekulare Pharmakologie, Institut für Pharmakologie and Klinische Pharmakologie, Universitätsklinkum Düsseldorf, Düsseldorf, Germany.
ABSTRACT
Remodeling of the dermal extracellular matrix occurs during photoaging. Here, the effect of repetitive UVB irradiation on dermal hyaluronic acid (HA) was examined. C57/BL6 mice were chronically (182 days) irradiated with UVB, and consecutive skin biopsies were collected during the irradiation period and afterward (300 and 400 days of age). UVB caused marked loss of HA from the papillary dermis and down- regulation of HA synthase 1 (HAS1), HAS2, and HAS3 mRNA expression. In contrast, hyaluronidases (HYAL) 1, HYAL2, and HA receptor CD44 were unchanged. Furthermore, transforming growth factor beta-1 (TGF-beta1) and TGF- beta1-receptor II expression were decreased in UVB-irradiated biopsies, and TGF- beta1 strongly induced HAS1 and HAS2 expression in cultured dermal fibroblasts. Therefore, TGF-beta1 might be one factor involved in UVB-induced down-regulation of HAS enzymes. In addition, total cell number and the percentage of proliferating fibroblasts in the papillary dermis of UVB-irradiated mice were decreased. Down- regulation of HAS2 by lentiviral overexpression of short hairpin RNA in vitro caused inhibition of HA synthesis, DNA synthesis, and migration of dermal fibroblasts. In conclusion, chronic UVB irradiation induces loss of HA from the dermis, thereby contributing to the quiescent phenotype of dermal fibroblasts
12. Cell Tissue Res. 2003 Mar;311(3):393-9. Epub 2003 Feb 25.
Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen.
Oesser S, Seifert J.,Surgical Research of the Department of General Surgery and Thoracic Surgery of the University of Kiel, Michaelisstrasse 5, 24105, Kiel, Germany
Abstract
The functional integrity of articular cartilage is dependent on the maintenance of the extracellular matrix (ECM), a process which is controlled by chondrocytes. The regulation of ECM biosynthesis is complex and a variety of substances have been found to influence chondrocyte metabolism. In the present study we have investigated the effect of degraded collagen on the formation of type II collagen by mature bovine chondrocytes in a cell culture model. The culture medium was supplemented with collagen hydrolysate (CH) and biosynthesis of type II collagen by chondrocytes was compared to control cells treated with native type I and type II collagen and a collagen-free protein hydrolysate. The quantification of type II collagen by means of an ELISA technique was confirmed by immunocytochemical detection as well as by the incorporation of (14)C-proline in the ECM after a 48 h incubation. Chondrocytes in the control group were maintained in the basal medium for 11 days. The presence of extracellular CH led to a dose-dependent increase in type II collagen secretion. However, native collagens as well as a collagen-free hydrolysate of wheat proteins failed to stimulate the production of type II collagen in chondrocytes. These results clearly indicate a stimulatory effect of degraded collagen on the type II collagen biosynthesis of chondrocytes and suggest a possible feedback mechanism for the regulation of collagen turnover in cartilage tissue.
13. Int J Dermatol. 1994 Feb;33(2):119-22.
Hyaluronic acid in cutaneous intrinsic aging.
Ghersetich I, Lotti T, Campanile G, Grappone C, Dini G., Department of Dermatology, University of Florence, Italy.
Abstract
BACKGROUND: In elderly individuals all components of the skin and subcutaneous tissue undergo histologic and ultrastructural changes. The turgidity of the dermis appears decreased, presumably due to altered patterns and levels of glycosaminoglycans (GAGS), especially hyaluronic acid and dermatan sulfate that are the most common. A linear, age-related decrease in the content of GAGS (mainly hyaluronic acid) has been hypothesized in human aged skin.
METHODS: We used the cationic dye Alcain Blue to selectively stain hyaluronic acid within the dermis in old and young subjects to compare ultrastructurally its topography and variations with age.
RESULTS: We demonstrated a progressive reduction in the number of electron- dense granules of hyaluronic acid and of their filaments until they were completely absent in subjects aged 60.
CONCLUSIONS: We propose that the variations of the levels of hyaluronic acid in the dermis in aging could account for some of the most striking alterations of the aged skin, including decreased turgidity, less support for microvessels, wrinkling, and altered elasticity.
14. Arthritis Rheum. 1998 May; 41(5):938.
Treatment of rheumatoid arthritis with oral Type II Collagen. Results of a multicenter, double-blind, placebo-controlled human clinical trial
Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, Fletcher MJ, Chasan-Taber S, Finger E, Morales A, Le CH, Trentham DE. Beth Israel Deaconess Medical Center, Boston, Massachusetts 02215, USA.
ABSTRACT
OBJECTIVE: Oral administration of cartilage-derived type II collagen (CII) has been shown to ameliorate arthritis in animal models of joint inflammation, and preliminary studies have suggested that this novel therapy is clinically beneficial and safe in patients with rheumatoid arthritis (RA). The present study was undertaken to test the safety and efficacy of 4 different dosages of orally administered CII in patients with RA.
METHODS: Two hundred seventy-four patients (274) with active RA were enrolled at 6 different sites and randomized to receive placebo or 1 of 4 dosages (20, 100, 500, or 2,500 microg/day) of oral CII for 24 weeks. Efficacy parameters were assessed monthly. Cumulative response rates (percentage of patients meeting the criteria for response at any time during the study) were analyzed utilizing 3 sets of composite criteria: the Paulus criteria, the American College of Rheumatology criteria for improvement in RA, and a requirement for > or = 30% reduction in both swollen and tender joint counts.
RESULTS: Eighty-three percent of patients completed 24 weeks of treatment. Numeric trends in favor of the 20 microg/day treatment group were seen with all 3 cumulative composite measures. However, a statistically significant increase (P = 0.035) in response rate for the 20 microg/day group versus placebo was detected using only the Paulus criteria. The presence of serum antibodies to CII at baseline was significantly associated with an increased likelihood of responding to treatment. No treatment-related adverse events were detected. The efficacy seen with the lowest dosage is consistent with the findings of animal studies and with known mechanisms of oral tolerance in which lower doses of orally administered autoantigens preferentially induce disease-suppressing regulatory cells.
CONCLUSION: Positive effects were observed with CII at the lowest dosage tested, and the presence of serum antibodies to CII at baseline may predict response to therapy. No side effects were associated with this novel therapeutic agent. Further controlled studies are required to assess the efficacy of this treatment approach.
15. J Cell Physiol. 1998 Dec;177(3):465-73.
Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix.
Greco RM, Iocono JA, Ehrlich HP., Department of Surgery, Hershey Medical Center, Pennsylvania State University College of Medicine, 17033, USA.
Abstract
Human dermal fibroblasts suspended in a collagen matrix exhibit a 4-day delay in cell division, while the same cells in monolayer divided by day 1. The initial rates of 3H- thymidine incorporation by cells in monolayer or suspended in collagen were not significantly different. When suspended in collagen, there was a threefold increase in the proportion of cells in a tetraploidal (4N) DNA state compared to the same cells in monolayer. Flow cytometry analysis and 3H-thymidine incorporation studies identified the delay of cell division as a consequence of a block in the G2/M of the cell cycle and not an inhibition of DNA synthesis. The inclusion of 150 microg/ml of hyaluronic acid (HA) in the manufacture of fibroblast populated collagen lattices (FPCL) caused a stimulation of cell division, as determined by cell counting; increased the expression of tubulin, as determined by Western blot analysis; and reduced the proportion of cells in a 4N state, as determined by flow cytometry. HA added to the same cells growing in monolayer produced a minimal increase in the rate of cell division or DNA synthesis. HA supplementation of FPCLs stimulated cell division as well as tubulin concentrations, but it did not enhance lattice contraction. The introduction of tubulin isolated from pig brain or purchased tubulin into fibroblasts by electroporation prior to their transfer into collagen lattices promoted cell division in the first 24 hours and enhanced FPCL contraction. It is proposed that tubulin protein, the building blocks of microtubules, is limited in human fibroblasts residing within a collagen matrix. When human fibroblasts are suspended in collagen, one effect of added HA may be to stimulate the synthesis of tubulin which assists cells.
List of Additional Supporting Studies




